Pt/NbPWO双功能催化剂的制备及氢解碱木质素制备芳香单体
来源:优秀文章 发布时间:2023-01-25 点击:
曹美芳,陈博,阮涛,欧阳新平,*,邱学青
1华南理工大学化学与化工学院广东省绿色化学产品技术重点实验室,广州 510640
2 广东工业大学轻工化工学院,广州 510006
Lignocellulosic biomass consisting of cellulose,hemicellulose and lignin is the abundant renewable resource,which is capable of substituting fossil resources in the production of chemicals and fuels1-3. However, lignin is notoriously recalcitrant to degradation, and hence is always treated as wastes4-6. If this resource can be efficiently transformed into chemicals or fuels, it can alleviate the dependence of fossil resources. Consequently, researches on lignin depolymerization have drawn an enormous amount of attention7,8.
Many approaches to the conversion of lignin have emerged over the past decade, including hydrogenolysis9, oxidative depolymerization10, biodegradation11, photocatalysis12etc.Hydrogenolysis of lignin is considered as an efficient reductive depolymerization strategy to produce bulk aromatic compounds.During this process, a majority of heterogeneous catalysts based on metal (Ru, Pt, Pb, Ni) have been developed12-14.
Niobium oxides as both promoter and support of catalyst can enhance catalytic activity and prolong catalyst life, which got lots of attentions in the catalytic transformation of lignin15.Wang’s group recently reported that layered Ru/Nb2O5could promote the cleavage of C-O bonds, and hence gain high yield of aromatic hydrocarbons16. Au/Nb2O5catalyst was also reported to be used in conversion lignin into phenolics, in which the synergistic effect of both the niobium support and electronrich Au nanoparticles facilitated the cleavage of C-O linkage17. Xia reported that woody biomass was directly converted to liquid alkanes over Pt/NbOPO4catalyst. The cleavage of C-O linkage was attributed to the synergetic effect of noble metal Pt particles which promote the dissociation of hydrogen and NbOxspecies which produce the Brönsted acid sites and trigger specific adsorption18. Lately, the cleavage mechanism of C-C bonds via NbOxspecies was illustrated by depolymerization of aromatic plastic waste and aromatic polymers such as polyethylene terephthalate, polycarbonate, and polyphenylene oxide etc10,19.
The current depolymerization of lignin is focused on cleavage of lignin β-O-4 linkages, resulting in lots of C-C linkages existed in the form of aromatic oligomers. Because the dissociation energy of C-C bonds (226-494 kJ·mol-1) is higher than that of C-O bonds (209-348 kJ·mol-1), the improved efficiency of depolymerization is dependent on the cleavage efficiency of C-C bond20,21.
To provide the efficient catalyst and realize the efficient valorization of lignin, an environmentally friendly strategy was used to construct a Pt/NbPWO bifunctional catalyst, in which the NbPWO carrier was prepared by hydrothermal recrystallization of microemulsion containing template and Nb precursor. This catalyst applied to the hydrogenolysis of alkali lignin contributed to a high yield of aromatic monomer. This work provides guidance to design catalyst and paves a way to the valorization of alkali lignin.
2.1 Catalyst preparation
The mesoporous niobium phosphotungstic acid (PW12/Nb2O5abbreviate the NbPWO) catalyst support was prepared via hydrothermal method. In briefly, 36 mL of 0.25 mol·L-1ammonium niobate oxalate hydrate (99.9%, Shanghai Aladdin Biochemical Technology Co., Ltd., China), 0 g, 1 g, 1.5 g, 2.0 g phosphor-tungstic acid (PW12) (99%, Shanghai Macklin Biochemical Co., Ltd., China) and 12 mL cetyl trimethyl ammonium bromide (CTAB) (99%) were mixed with constant stirring rate of 300 r·min-1(named: PW0, PW1, PW2, PW3).Deionized water was then added to this solution in order to adjust pH. The emulsion was stirred for 30 min, followed by kept in an oven at 80 °C for 24 h, then aged at 130 °C for 24 h in a hydrothermal Teflon lined autoclave (HTG-100-SS1, Anhui CHEM Co., Ltd., China). The resultant solid was filtered,washed and dried, and then calcined in an air atmosphere22.According to the above preparation conditions, PW12was replaced with ammonium hydrogen phosphate (99%) and ammonium paratungstate (99%), named NbPO and NbWO.Additional, according to the above preparation conditions,ammonium niobate oxalate hydrate was replaced with aluminium trichloride (99%), colloidal silica (99%), titanium chloride (99%) and zirconium nitrate pentahydrate (99%),respectively, named AlPWO, SiPWO, TiPWO and ZrPWO.
Pt supported on NbPWO catalyst was prepared by wet impregnation. 0.3 g support and 0.5 mL of 1 mg·L-1chloroplatinic acid (99.9%, Shanghai Aladdin Biochemical Technology Co., Ltd., China) solution was added to 20 mL distilled water and stirred for 24 h. Then, this solution was heated at 80 °C vaporate the water under magnetic agitation. The samples were reduced in 8% H2/92% Ar atmosphere at 200 °C for 2 h with a heating rate of 3 °C·min-123.
2.2 Reaction procedure
The reaction for the hydrogenolysis of lignin was test as follow method: 0.1 g alkali lignin (99%, Sigma-Aldrich, UK)and catalyst (0.05 g) were loaded into an autoclave reactor (Auto Chem100, Beijing Century Senlong experimental Co., Ltd.,China) with 15 mL distilled water and 5 mL cyclohexane(99.5%). After sealed and purged, the reactor was charged with different pressure H2and conducted at 300 °C with a magnetic stirring speed of 400 r·min-1, for 4 h. After the reaction, the catalyst was separated the organic phase was extract using ethyl acetate. The products were analyzed by gas chromatography(GC 2015, Shimadzu, Iapan) and GC-MS (5975C-7890A,Agilent Technology Co., Ltd., Germany)24. The aromatic product yield for hydrogenolysis of alkali lignin was calculated as follows:

Structural information of depolymerized products and native lignin was analyzed by two-dimension heteronuclear single quantum coherence (HSQC)25. The molecular weight of depolymerized products and native lignin was calculated by gel permeation chromatography (GPC)26.
2.3 Catalyst characterization
X-ray diffraction (XRD): XRD measurement for the state and crystal structure of elements in the catalyst by Bruker D8 Advance (D8 Advance, Bruker Co., Ltd., Germany). The test conditions: Cu Kαwas used as the incident light source, the working voltage was 40 kV, the working current was 40 mA, the scanning speed was 10 (°)·min-1, and the scanning Angle was 10°-80°. MDI Jade 6.0 software was used to analyze the data27.
Inductively coupled plasma atomic emission spectroscopy(ICP-AES): The content of precious metals in the catalyst were determined using ICP-AES (Agilent 5110, Agilent Technology Co., Ltd., Germany)28.
Brunauer Emmett Teller (BET): The specific surface area and pore size distribution of catalyst were determined by nitrogen adsorption-desorption isotherms (Tristar 3020, Mike Co., Ltd.,USA). BET equation was used to calculate the specific surface area of the sample, and BJH model was used to calculate the pore distribution27.
Infrared spectra of adsorbed pyridine (Py-IR): The sample was weighed and pressed into thin slices, placed in the sample chamber of the infrared spectrometer (Tensor 27, Bruker Co.,Ltd., Germany). After vacuum pretreatment, the background spectrogram was collected. Pyridine was then adsorbed for 30 min, and desorbed and IR scanned after equilibrium26.
Ammonia temperature programmed desorption (NH3-TPD):Qualitative and quantitative analysis of solid acid catalyst was carried out via NH3-TPD (Auto Chem 2920, Mike Co., Ltd.,USA). Sample was placed in a reaction tube, and the temperature was raised from room temperature to 400 °C at 10 °C·min-1for drying pretreatment. After He was purged for 1 h, the sample was cooled to and exposed to 10% NH3/He for 1 h until saturation.Finally, the desorption gas was detected by TCD detector at a temperature of 10 °C·min-1to 700 °C.
X-ray photoelectron spectroscopy (XPS): Valence state of different elements was analyzed by XPS (Thermo Scientific,Thermo Fisher Co., Ltd., USA)29. The full and partial spectra of different elements were obtained by calibration with the binding energy of C 1s (284.8). Then peak separation, fitting and integration of spectra of different elements used Advantage software.
Hydrogen temperature-programmed reduction (H2-TPR):Reduction degree of precious metal and its interaction with support were performed using the Auto Chem 2920 apparatus(Auto Chem 2920, Mike Co., Ltd., USA). The above samples were heated to 900 °C at a heating rate of 10 °C·min-1and amount of hydrogen consumption was detected using a TCD detector30.
3.1 Catalyst characterization
Fig. 1a showed schematic representation of the formation process of the flower-shaped NbPWO support. The hydrothermally synthesized flower-like assembled nanorod NbPWO sample contained stacked nanorods, which arranged together and formed a spherical micro-flower, as confirmed by SEM and TEM (Fig. 1b,d). SEM image showed a developed channel structure on flower-shaped NbPWO support, which could promote diffusion of lignin bio-oil. Pt loaded by wet impregnation and the loading amount was 3% (w, mass fraction)by ICP-AES. EDS spectra showed the presence of Pt, Nb, P, W and O elements in Pt/NbPWO. Owing to the special flowershaped structure, NbPWO support could not only make full use of 3D rods for the loading of the active Pt metal, but also effectively limit the aggregation or restacking of nanorods.
The X-ray diffraction (XRD) patterns of Pt/NbPWO display typical peaks at 2θ = 23.1°, 23.7°, 33.5° and 33.9° etc., which are readily indexed to planes of polycrystalline structure PW phase (PDF#41-0326) (Fig. 2a). Additionally, diffraction peak of Pt/NbPWO was in good agreement with PW phase, indicating the related crystal structure. No diffraction peak from NbOxis found in Pt/NbPWO composite because of the amorphous state of NbOx. This result was well matched with EDX mapping images (Fig. 1c), implying the homogeneous dispersion of NbOxthroughout the PW12framework. In general, we deduced that the Keggin structure of PW12remained unchanged when it was introduced into NbOxby hydrothermally method31,32.
The N2adsorption isotherm measure of Pt/NbPWO showed an obvious hierarchically porous structure with a significantly higher specific surface area of 74 m2·g-1and a total pore volume of 0.19 m3·g-1(Fig. 2b and Table 1) than Pt/NbPO and Pt/NbWO, which is possibly due to that the addition of PW12promoted the formation of nano-flower structure of the support.NbPWO was a heteropolyacid composed of heteropoly anions(PW12) and cations (NbOx). PW12was formed by coordination bridge of oxygen atoms and had a certain pore structure (Fig.1a).
Fig. 2c showed the acid content of NbPWO support at different calcination temperatures (500 °C, 600 °C, 700 °C). It was worth noting that the acid content was as higher as 1 mmol·g-1when the calcination temperature was 500 °C. With the increase of calcination temperature, the catalyst of solid acid content decreased obviously. When the calcination temperature is 700 °C, the acid content of the catalyst is 0.4 mmol·g-1. The reason may be that the crystal system of NbOxwas from T-NbOxto TT-NbOxat high calcination temperature30.

Fig. 1 (a) Schematic representation of the formation process of the flower-shaped NbPWO support after heat treating in air at 500 °C.(b) SEM image, (c) the HADDF result and corresponding EDX mapping images and (d, e) TEM images of Pt/NbPWO samples.

Fig. 2 (a) XRD pattern of Pt/NbWO samples and PW12 and (b) N2 adsorption isotherms of Pt/NbWO, Pt/NbPO and Pt/NbPWO; (c) NH3-TPD curves of different calcination temperature.
Py-IR experiments were conducted to determine the acidic properties and the reaction mechanism in hydrogenolysis. Fig. 3 displayed that intense bands of Pt/NbPWO catalyst at 1450 cm-1and 1610 cm-1were assigned to Lewis acid (L), at 1543 cm-1and 1575 cm-1were attributed to Bronsted acid (B) and at 1492 cm-1was ascribed to the synergic effect of L and B acid sites15.Table 1 showed the content of L and B acid respectively in the above three Nb-based samples. The acid contents of the Pt/NbPWO catalyst were significantly higher than those of the other catalysts. In hydrogenolysis of lignin, L acid could promote C-O bonds cleavage, while B acid could promote C-C bonds fracture13. Pt/NbPWO catalyst had abundant B acidsites and higher acid content, which could effectively transform alkali lignin, and improve the yield of aromatic monomer.

Fig. 3 Py-IR spectra of Pt/NbWO, Pt/NbPO and Pt/NbPWO.
Table 1 Pore size distribution and acid content of different catalysts.
Fig. 4 shows the H2-TPR patterns of the Pt/NbPWO, Pt/NbPO and Pt/NbPWO. The supports possess obvious reduction peaks,which could be ascribed to the bulk oxygen and surface or subsurface oxygen of NbPWO, NbPO and NbPWO33. Furthermore,two peaks at about 93 °C and 160 °C (< 200 °C) should attribute to the bulk platinum oxide reduction (PtOxto Pt0)34. Reduction peaks of Pt0over Pt/NbPWO was more obvious than those of other catalysts, the reason may be that flower -shaped Pt/NbPWO catalyst promoted the loading of the active Pt metal.A low valence of NbOxappeared after the reduction of Pt/NbPWO at 450 °C, while the additional weak reduction peak near 395 °C was assigned to the reduction of surface NbPWO which interacted with Pt, implying a stronger metal-support interaction (SMSI) of Pt particles with the NbPWO support35.These results demonstrated the existence of the Pt-NbOxinterface, where the NbPWO surface provided more anchoring sites for Pt.
To explore the surface valence state of catalysts, the XPS survey spectrum of Pt/NbWO, Pt/NbPO and Pt/NbPWO samples were presented in Fig. 5. The Pt 4forbital showed two peaks in above samples, which were attributed to Pt 4f7/2and Pt 4f5/2,respectively. The binding energies (BEs) of Pt0were determined as 71 eV and 74 eV36. When P together with W species participated in the synthesis of catalyst, the BEs of Pt was significantly smaller compared with those with sole P and W species. The downward shift in BEs indicated that PW12affected the electron cloud density and distribution of Pt nanoparticles in the catalyst, reducing the energy barriers of H2dissociation. As shown in Fig. 5b, the Nb5+also presented two peaks corresponding to Nb 3d5/2and Nb 3d3/2. The binding energies of Nb5+over the Pt/NbPWO obviously decreased, indicating that PW12could promote the formation or exposure of Nbδ+species and increase surface acidic sites.

Fig. 5 XPS spectra of (a) Pt 4f orbital in NbWO, NbPO and NbPWO. and (b) Nb 3d orbital in Pt/NbWO, Pt/NbPO and Pt/NbPWO.

Fig. 6 (a) Reaction results for the hydrogenolysis of alkali lignin over various Pt-loaded catalysts, influence of (b) temperature and(c) H2 pressure over Pt/NbPWO. (a) Reaction conditions: alkali lignin 0.1 g, catalyst 0.05 g, H2O 15 mL, cyclohexane 5 mL, 300 °C, H2 1.2 MPa,4 h. (b) Reaction conditions: alkali lignin 0.1 g, catalyst 0.05 g, H2O 15 mL, cyclohexane 5 mL, H2 1.2 MPa, 4 h. (c) Reaction conditions: alkali lignin 0.1 g, catalyst 0.05 g, H2O 15 mL, cyclohexane 5 mL, 300 °C, 4 h. (d) Main structure of the depolymerized products.
3.2 Evaluate of catalytic performance
Fig. 6a compared the hydrogenolysis performance for alkali lignin over various Pt-based catalysts under the same reaction condition. It is found that among the selected Pt catalysts,Pt/NbPWO exhibited the highest yield of aromatic monomers(18.04%). In comparison, commercial Pt/C catalyst only gave a very low aromatic monomer yield (8.13%). The reason may be ascribed to the acid sites of Pt/NbPWO, preventing further hydrogenation24. Comparing with Pt/NbPO and Pt/NbWO,Pt/NbPWO possessed larger specific surface area and abundant mesoporous showed a large enhancement of catalytic activity.Moreover, the special flower-shaped structure took advantage of 3D rods for the loading of the active Pt metal, making Pt species uniform distributed on the NbPWO catalyst (Fig. 1c) and could effectively limit the aggregation.
As shown in Fig. 6, the influence of reaction temperature and H2pressure on catalytic depolymerization of alkali lignin over Pt/NbPWO was studied. The yield of the aromatic monomers over the Pt/NbPWO catalyst increased to the peak value of 18.05% at 300 °C and 1.2 MPa H2. The efficiency of lignin depolymerization decreased under a low H2pressure short after reaction temperature due to the recalcitrance and the stability of lignin. However, increasing the H2pressure caused low aromatic monomer yield, the reason may be ascribed to overhydrogenation of benzene ring and partial products dissolved in aqueous-phase. Increasing the reaction temperature also caused low aromatic monomer yield, which may be attributed to the recondensation of lignin-depolymerized monomer products19.
The depolymerization products of alkali lignin were analyzed by HSQC (Fig. 7 and Table 3). The signals of Aα(δC/δH 71.4/4.87 and δC/δH 85.5/4.7) and Aβ(δC/δH 84.1/4.32 for G unit and δC/δH 87.1/4.11 for S unit) corresponded to benzylic alcohol. The signals of Bα(δC/δH 876.7/5.5), Bβ(δC/δH 53.7/3.1), and Bγ(δC/δH 62.9/3.7) corresponded to phenylcoumaran linkages. The signals of Cα, Cβand Cγ(δC/δH 85.6/4.7, δC/δH 54.5/2.9 and δC/δH 72/3.8, 4.2, respectively)corresponded to resinol linkages13. The aromatic region of alkali lignin is mainly composed of G type units along with a small amount of S and H types37. Compared with the alkali lignin, the peak area of A, B and C structures of the depolymerized product decreased significantly or the signal peak disappeared directly,indicating that the catalyst could effectively break the C-O and C-C bonds of alkali lignin37.
Fig. 8 shows that the molecular weight of alkali lignin was 1379 Da, which indicated that depolymerization products without the addition of catalyst was 727 Da, whereas the molecular weight of depolymerization products with Pt/NbPWO catalyst reduced to 583 Da, confirming that the catalyst could effectively break the linking bonds of lignin subunits.
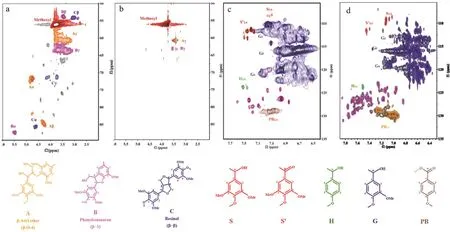
Fig. 7 2D-HSQC NMR spectra of alkali lignin (a: side-chain region; c: aromatic region) and depolymerization products at optimized condition(b: side-chain region; d: aromatic region); (A) β-O-4 alkyl-aryl ethers; (B) phenylcoumarans; (C) resinols; (S) syringyl units; (S’) oxidized syringyl units; (G) guaiacyl units; (H) P-hydroxyphenyl units and (PB) coumarate.

Fig. 8 GPC distribution of depolymerization products.
In order to verify the universality of the catalyst, Pt/NbPWO catalyst was applied to the depolymerization of Birch organosolv lignin (BOSL) and enzymatic hydrolysis lignin (EHL). The products distributions were shown in Table 2. The depolymerization products of the three kinds of lignin were mainly G-type products, with a small amount of H-type and S-type products37. The yield of aromatic monomer of lignin extracted with dioxane was 35.17% and that of enzymatic hydrolysis was 25.31%. The structures of native BOSL and EHL were measured by two-dimensional HSQC NMR spectroscopy(Fig. 9). Compared with alkali lignin, the β-O-4 units correlation signals of BOSL and EHL obviously resonated, meantime, the β-O-4 bonds contents of BOSL and EHL were higher (Table 3),which suggested the chemical structure changed mainlyviahydroxyl condensation of alkali lignin in the extraction process.The β-O-4 bond contents of BOSL were higher than that of AL and EHL, hence gaining the higher yield of aromatic monomers.By comparing the aromatic monomers yield and total bond content of different original lignin, it was found that utilization ratio of AL and EHL was higher over Pt/NbPWO catalyst, the reason may be that the structure of BOSL was fragile due to more β-O-4 units, causing that the depolymerization product was condensed.

Table 2 The hydrogenolysis of lignin into aromatic monomers in different condition.

Table 3 Structural characteristics of the different lignin by 2D-HSQC method.
3.3 Catalyst reusability
Stability of catalyst was shown in Fig. 10a, which indicated that the catalyst still maintained a high lignin depolymerization efficiency after used 5 times. XRD profiles of the used and fresh Pt/NbPWO catalysts (Fig. 10b) indicated that the crystal structure of the catalyst was not significantly changed. The particle size of NbOxon the used Pt/NbPWO catalyst was slightly larger relative to that of fresh activated samples,indicating that catalyst particles tiny agglomeration during the hydrothermal reaction (Fig. 10b). NH3-TPD profiles (Fig. 10c)shown that the total acid content of Pt/NbPWO catalysts after 5 times slightly decreased from 1.08 mmol·g-1to 1.02 mmol·g-1.

Fig. 9 2D-HSQC NMR spectra of (a, b) BOSL and (c, d) EHL.

Fig. 10 (a) The stability of the Pt/NbPWO catalyst for the hydrogenolysis of alkali lignin. Reaction conditions: alkali lignin 0.1 g, catalyst 0.05 g,H2O 15 mL, cyclohexane 5 mL, H2 1.2 MPa, 300 °C for 4 h. (b) XRD analysis and (c) NH3-TPD profiles of the fresh and used Pt/NbPWO catalyst.
The Pt/NbPWO catalyst was prepared by hydrothermal and wetness impregnation methods. The Pt/NbPWO catalyst displayed good ability for the cleavage of C-O ether band and C-C bonds of lignin, giving 18.05%, 35.17% and 25.13% of aromatic monomer yields for alkali lignin, BOSL and EHL,respectively. It was found that 500 °C was the optimized calcination temperature for preparing the catalyst, in which higher temperature led to a considerable loss of acidity, while lower temperature caused the unstablization of catalyst during the depolymerization process. Abundant Brønsted acid sites and high total acid content should contribute to the desired catalytic activity in the hydrogenation of lignin.
猜你喜欢 陈博华南理工大学新平 敢做,但要脚踏实地地做民生周刊(2022年14期)2022-07-08幼儿园里欢乐多幼儿园(2021年18期)2021-12-06小蚂蚁去游玩幼儿园(2021年16期)2021-12-06策划师名家名作(2021年1期)2021-11-13老腔唱新歌金秋(2021年22期)2021-03-10船舶导管螺旋桨声学处理研究与应用广东造船(2020年3期)2020-07-22让蘑菇幼儿园(2020年3期)2020-03-27梁文峻、巫金隆、黄靖鸿、吴国杰作品美与时代·城市版(2019年8期)2019-11-04人工智能方向赛题二《文化传承—汉字书法多场景识别》软件和集成电路(2019年5期)2019-07-31咸的“糖”作文大王·低年级(2019年5期)2019-06-13推荐访问:制备 木质素 催化剂