Nucleation,and,growth,behavior,of,coating,film,on,Mg-Al-Zn,alloy,with,different,surface,topographies,via,plasma,electrolytic,oxidation
来源:优秀文章 发布时间:2022-12-10 点击:
Nisa Nashrah,Sung Hun Baek,Young Gun Ko
Materials Electrochemistry Group,School of Materials Science and Engineering,Yeungnam University,Gyeongsan 38541,Republic of Korea
Abstract This work was made to investigate how nucleation and growth behavior of the coating film were affected by surface topographies of Mg-Al-Zn alloy substrate during the initial stage of plasma electrolytic oxidation (PEO).To satisfy this end,a single substrate was prepared by mechanical treatment exhibiting rough and smooth regions with an equal area on the surface.The rough region with prominent hills and grooves induced the breakdown of passive film,which was indicated by an early appearance of plasma discharge on the rough region since nucleation of coating film occurred preferentially around the hills.However,the coating film grown on the grooves was somewhat thicker and more porous than the film grown on the hills and smooth regions.This was due to the fact that the growth of the coating film was found to be localized in the presence of rough region,which was in line with the discharge activities.Herein,the nucleation and growth behavior during the initial stage of PEO will be discussed schematically on the basis of microstructural interpretation.
Keywords: Mg alloy;Surface roughness;Plasma electrolytic oxidation;Coating film.
Mg and its alloys have been of great interest for miscellaneous applications ranging from automobile manufacturing to bio-implant engineering for their high electromagnetic shielding,good specific strength,and acceptable biocompatibility [1-3].Nevertheless,poor corrosion resistance remains a major shortcoming,which limits their practical implementation [4,5].Efforts to address such drawback have developed protective coating via surface modification,including chemical conversion coating,anodizing,and plasma electrolytic oxidation (PEO) [6-8].PEO,where the metallic surface was reformed through plasma-assisted electrochemical reaction in an ecofriendly electrolyte,was favorable for growing a thick coating layer which adhered strongly on to the metallic substrate [9,10].
Previous investigations of PEO on Mg alloys show that the microstructure of the coating film varies with processing variables,such as electrolyte composition,electrical regime,and substrate condition [11-16].Among them,substrate features,such as its grain size and intermetallic phase have been reported to induce different growth patterns of the coating film on the surface [17,18].In addition,a modification in the surface structure prior to PEO was also reported to influence the microstructure of the final coating film.For instance,Huang et al.[19] demonstrated the effect of micro-grooving on a YL113 Al substrate prior to 150min of PEO treatment.The coating film formed on the grooved sample,whose surface roughness was 0.6μm,was thicker than that formed on the non-grooved sample.This was attributed to an increase in the partial growth rate of the coating film from 0.66 to 1.0 μm·cm-1by the presence of the grooved structure [19].Another study reported by Zhu et al.[20],showed that the variation in the surface roughness of Ti-6Al-4V alloy significantly altered the structural features of the coating film formed on the substrate [20].A decrease in surface roughnessmay reduce Gibbs free energy,which facilitated the homogeneous growth of the coating film and hence suppressed the formation of micropores.Consequently,the sample with the lowest roughness produced a coating film with a relatively compact structure.Most recently,PEO treatment of an Al alloy with a trapezoidal structure,produced by high-precision machining,resulted in a coating film with large micropores and visible microcracks.However,the hardness of the sample with trapezoidal structure was~1.3 times higher than that of the sample with a nearly flat structure.This outcome was assigned to the high content of crystallineα-Al2O3,ɣ-Al2O3,and 3Al2O3·2SiO2,in the final coating induced by the high temperature and stress concentration at the sharp edge of the trapezoidal interface [21].Studies suggested that an increase in surface roughness results in a thick yet porous coating film,whereas with decreasing surface roughness,a coating film with a more compact structure is obtained.
While it is accepted that the surface roughness of the substrate may control the final microstructure of the coating films,its role in determining the nucleation and growth phenomena during the initial stage of PEO remains unclear.Moreover,previous studies have only focused on the variation of surface roughness between different substrates,while the use of a single substrate to maintain the consistency of the main variable should provide a facile approach beneficial for investigating the nucleation and growth process in a direct manner.
Thus,the present study aims to evaluate the nucleation and growth behavior of a coating film formed on a single Mg-Al-Zn alloy substrate having two different surface structures together during the initial stage of PEO.The nucleation and growth phenomena of the coating film are discussed based on microstructural interpretation.The corrosion assessment by means of potentiodynamic polarization test is used to describe the polarization behavior of the coating film related to surface roughness.
A commercial Mg-Al-Zn alloy purchased from POSCO(Republic of Korea) was used as the substrate in this study without further processing prior to PEO.As we confirmed from the manufacturer,the substrate consisted of 2.89 Al,0.96 Zn,0.31 Mn,0.15 Fe,0.12 Si,and balanced Mg (in wt.%)which was listed in Table 1.The sample having a dimension of 15 (length)×25 (width)×4 (thickness) mm3worked as an anode and a stainless steel wire was employed as a cathode.The sample with a dimension of 15 (length)×25 (width)×4(thickness) mm3worked as an anode and a stainless steel wire was employed as a cathode.The sample was polished mechanically with emery paper of grade 2400 to obtain a smooth finish.The half surface of the substrate was grinded again using emery paper of grade 80 to produce rough and smooth regions of equal area.The rough and smooth regions are denoted as Rr and Sr,respectively.Surface topographies of the initial substrate was evaluated by atomic force microscopy (AFM,Xe-100).An arithmetic mean roughness (Ra)used commonly was chosen to quantify the surface roughness of the present samples by means of non-contacting assessment ‘across’ the geographical structure.TheRavalues was measured by the Eq.(1) below [22],

Table 1 Chemical constituent of the initial substrate used in PEO treatment.

Table 2 Pote ntiodynamic polarization results of Sr-G,Rr-G,Sr-N,and Rr-N samples measured in 3.5wt.% NaCl solution.The scan was from -0.25 to 0.4V vs.OCP at a scan rate of 1 mV·s-1.

Fig.1.Representative AFM images of an initial sample taken from two areas showing (a) rough and (d) smooth region,respectively.

where Z(x) is a function representing surface roughness and l is a measurable length.TheRavalues of rough and smooth region calculated based on AFM measurement were found to be~1 and~0.03μm,respectively as shown in Fig.1.Prior to use,the sample was rinsed with distilled water,cleaned ultrasonically with acetone,and dried in a stream of warm air.Subsequent PEO treatments were conducted in an aqueous electrolyte containing KOH and Na2SiO3at a concentration of 0.1M and 0.05M,respectively (pH: 11.83,conductivity:12.39 mS·cm-1) with current density of 50 mA·cm-2and frequency of 60Hz.Details of the experimental design can be found elsewhere [23].
In this study,the nucleation and growth stages were distinguished based on the characteristics of plasma discharges.The first appearance of plasma discharges was considered the nucleation stage (N),which were identified at 20 and 25s for Rr and Sr,respectively.The proliferation of plasma discharges up to 40s was considered the growth stage (G).Accordingly,the Rr and Sr at the nucleation and growth stages are denoted as Rr-N,Sr-N,Rr-G,and Sr-G,respectively.The visual appearance of the plasma discharges on the sample surface was captured with a digital single-lens reflex camera(Canon EOS-700D) over time,and the intensity of the plasma discharges was analyzed using image analysis software.The morphological surface and cross section of the coating film were identified by field-emission scanning electron microscopy (FESEM;S-4800,Hitachi) to study the nucleation and growth phenomena of the coating film.
The polarization behavior of the coating film were evaluated through potentiodynamic polarization test using a potentiostat (Interface 1000,Gamry Instruments) in a 3.5wt.% NaCl solution.To study the polarization behavior related to surface roughness,four samples were used,namely Rr-N,Sr-N,Rr-G,and Sr-G.The assessment was carried out in three-electrode system,consisting of each sample as the working electrode,Pt as counter electrode,and Ag/AgCl as reference electrode.Prior to polarization test,each sample was immersed in the solution for 1h until reaching a steady potential at open circuit.During potentiodynamic polarization test,the sample was scanned from -0.25 to +0.4V with respect to the open circuit potential at a scan rate of 1 mV·s-1.Each assessment was repeated at least three times to ensure that the data obtained would be feasible.Electrochemical parameters,such as the corrosion current density (icorr),corrosion potential (Ecorr),and slopes of the anodic and cathodic reactions (βaandβc,respectively),were iterated through Tafel extrapolation.The polarization resistance (Rp)was calculated using the representative equation proposed by Stern-Geary [24] shown in Eq.(2) below

3.1.Plasma discharges at the initial stage of PEO
The occurrence of plasma discharges during the initial stage of PEO was influenced by the initial surface topographies of the Mg-Al-Zn alloy substrate.The effect of surface roughness on the behavior of plasma discharges was evaluated at the onset of breakdown and during proliferation by considering voltage-time curve shown in Fig.2.
3.1.1.Initiation of plasma discharges at the onset of breakdown

Fig.2.Plot of the responding voltage vs.coating time of an Mg-Al-Zn substrate with rough and smooth regions during PEO at 50 mA·cm-2 for 60 s.The insets show the surface of the substrate along with the appearance of plasma discharges at different coating times,and their intensity profiles.
Fig.2 displayed the voltage-time curve of the sample,which consisted of rough and smooth regions coated by PEO at 50 mA·cm-2for 60s.Several images were provided as insets in Fig.2,showing the real image of the present sample prior to PEO and the appearance of plasma discharges at 20 and 40s during PEO along with the line graphs demonstrating a number of discharges as a function of intensity.From the voltage-time curve,two stages of PEO were identified based on the behavior of the responding voltage.Stage I was characterized by a linear increment in the responding voltage due to an increase in electrical resistance of the passive film growing on the substrate under constant current condition,consistent with the linear relationship of Ohm’s law.The linear increase in voltage continued until the resistance of the passive film was unable to sustain the intensive electric field,resulting in the breakdown of the passive film.The initial breakdown of the passive film occurs at~20s,upon reaching~227V,i.e.,breakdown voltage,which was accompanied by the appearance of plasma discharges across the substrate surface.This event indicated a transition from the conventional anodic oxidation in stage I to PEO in stage II.As shown in the inset of Fig.2;the plasma discharges observed in the Rr (left) at~20s preceded the appearance of plasma discharges in the Sr(right)at~25s.The difference in the ignition times of plasma discharges could be attributed to the nonhomogeneous distribution of the electrical field across the substrate-electrolyte interface,which suggested that the electrical resistance of the preformed passive film differed between regions.
According to the breakdown theory of Ikonopisov [25],initial breakdown took place through the defects in the passive film due to their relatively low electrical resistance.In this study,considering the high level of surface irregularities in the Rr,the electrical resistance of the passive film formed in this region was possibly lower than that of the passive film formed in the Sr.Consequently,the passive film in the Rr of the substrate would be inclined to experience an early breakdown.
After the initial breakdown,the slope of the responding voltage in stage II is significantly lower than that in stage I because the growth of the coating film relies on the activity of plasma discharges.
3.1.2.Proliferation of plasma discharges
The activity of plasma discharges intensified after 40s,the behavior differed between two regions of the present substrate.It was apparent that the discharge activities in the Rr were localized in some areas,while a uniform distribution of plasma discharges appeared across the Sr of the substrate.To analyze such characteristic of the plasma discharges,the graph in the inset of Fig.2 presented the number of plasma discharges as a function of their intensities.The associated number of plasma discharge and their intensities observed in Rr of the sample were higher than those observed on the Sr at 40s.Unlike in the Rr of the substrate,the low incidence of surface irregularities in the Sr maintained a homogeneous distribution of the electrical field,hence inhibited localization of breakdown spot.
Troughton et al.[26] postulated that plasma discharges might be sustained in some local areas containing defects until the defects are sealed completely.It is,accordingly,presumed that plasma discharges should be concentrated in certain locations of the rough region owing to the presence of numerous hills and grooves (i.e.,structural defects).This behavior was demonstrated by the plasma discharges apparent in the left region of the substrate surface,after 40s of PEO as displayed in the inset of Fig.2.
Our research suggested that the plasma discharges exhibited different characteristics not only during ignition but also during their proliferation.Such a difference in the manifestation of the plasma discharges was expected to influence the microstructure of the coating film that would explain its nucleation and growth phenomena.
3.2.Nucleation and growth behaviors of coating flim considering surface roughness
The nucleation and growth behaviors of the coating film were affected by the surface roughness of the initial substrate due to the difference in micro-regional characteristics of the passive film formed on the substrate,where the presence of grooves in the Rr triggered the localized growth of the coating film.Both the nucleation and growth phenomena could be explained by surface and cross-sectional morphologies of the coating film grown on both rough and smooth regions.
3.2.1.Effect of surface roughness on coating flim formation
Fig.3 shows the microstructures of the coating film formed on the rough and smooth regions of the substrate at the onset of breakdown.The surface morphology of the coating film in the Rr shown in Fig.3a,was characterized by two areas i.e.,hills and grooves.The surface of the Rr was not covered evenly by the coating film;the hills appeared to be coated,whereas the coating on the grooves was incomplete.In contrast,the surface of the Sr was coated uniformly,as depicted in Fig.3b.These observations were confirmed by the cross-section images presented in Fig.3c and d.As seen in Fig.3c,the oxide debris nucleated on the hills,suggesting that the breakdown initiated on the hills earlier than in the adjacent grooves,which resulted in an incomplete coating film.Meanwhile,as seen in Fig.3d,a thin coating film with a thickness of~1μm was detected on the Sr,which was possibly the result of homogeneous nucleation induced by a uniform passive film preformed on the smooth surface.
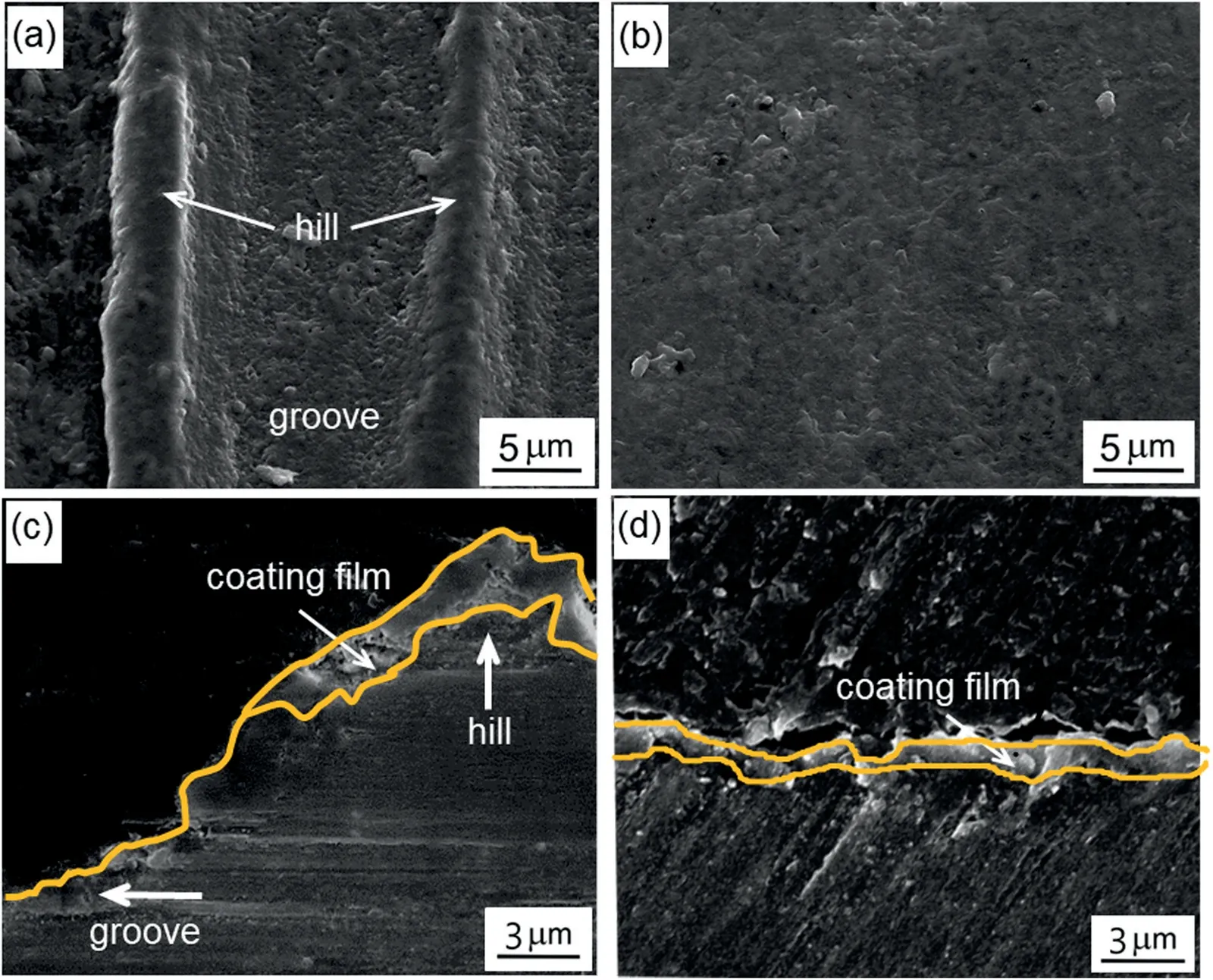
Fig.3.Representative SEM images showing the (a),(b) surface and (c),(d)cross-section morphologies of the nucleation stage at 20 and 25s in the rough and smooth regions,respectively.
In general thin film theory,the variation in the surface structure of the substrate led to inconsistent morphological features,including microstructure and thickness of the film formed on the substrate.If the substrate was rough,the surface diffusion of ionic molecules adsorbed to the interfaces of the solid material could exhibit different behaviors as a result of structural gradient [27].The correlation between the surface geometry of the metallic substrate and its affinity towards ionic molecules in the wet electrolyte was reported by Zuo et al.[28].They exposed 316L stainless steel,with different surface finishes,to a 0.01M NaCl solution to evaluate pitting corrosion.Depending on the surface roughness,the aspect ratio of the surface grooves,which was defined as the width (w) to depth (d) ratio,influenced the chemical reaction between the substrate and ionic species in the electrolyte.They suggested that the lower the aspect ratio (w/d),the greater the localized decay of the metallic substrate owing to the higher surface area [28].The decay of metallic materials during pitting corrosion and the formation of a passive film during PEO were expected to occur in a similar manner,in terms of the reaction between the metallic substrate and the electrolyte in solution [29,30].Considering the fact that the grooves possessed a higher surface area than the hills,the chemical reaction was expected to be concentrated in the former,resulting in a passive film that was presumably thicker than that formed on the hills or on the Sr.As a result,the distribution of the electric field was likely non-uniform and possibly concentrated around the hills coated with the thinner passive film.This triggered avalanches of electrons to move across the hills with ease,igniting plasma discharges.
Thus,it was suggested that the high surface roughness induced an early breakdown due to the nonuniform thickness of the passive film exhibiting nonhomogeneous electrical resistance.Since the nucleation phenomena in both regions were found to be directly affected by surface roughness,the difference in growth behavior of the coating film was expected.
Fig.4 presents the morphological structure of the coating film after~40s of PEO,where the plasma discharges participated in the growth stage.Fig.4a and b showed the surface morphologies of the coating film grown in the Sr and Sr,respectively.As seen in Fig.4a,the topographical features that defined the hill and groove areas were still evident after~40s of PEO treatment.It was apparent that the distribution of micropores in the coating film in the Sr was not uniform,i.e.,numerous micropores of different sizes were detected in the film grown on the groove,while the film grown on the hill was relatively compact.In contrast,a uniform film covered the Sr,as depicted in Fig.4b.These results were evident in the cross-sectional morphologies shown in Fig.4c and d.Although nucleation was first observed on the hills,the coating film grown on the grooves appeared thicker and more porous than that grown on the hills after~40s of PEO(Fig.4c).However,across the Sr,continuous homogeneous nucleation produced a coating film with a relatively compact structure and thickness of~2.5μm (Fig.4d).The morphologies of coating films produced through PEO were typically microporous or relatively compact structures [31,32].These microstructures of the coating films were considered the fingerprints of the discharge activities,where each pore was associated with a discharge phenomenon that occurred during PEO [33,34].In the case of the Rr,the activities of the plasma discharges were expected to be more pronounced in the grooves considering the numerous micropores identified in the film grown on the groove.This result was consistent with the nonuniform distribution of plasma discharges in the Sr at~40s,as shown in the inset of Fig.1.Although the nucleation of the coating film in the Sr occur on the hills was earlier than that on the grooves,the thickness of the coating film formed in the Sr was relatively comparable to that formed in the Sr after 40s of PEO.This was attributed to the fact that the coating film on the groove was moderately thicker than that on the hills because of the higher growth rate of the coating film formed in the Rr than that in the Sr.Such different growth rates corresponded to the intense plasma discharges observed concentrated in the grooves with a thinner coating film since nucleation first occurred on the hills,as evidenced in Fig.3c.
3.2.2.Proposed mechanism
All microstructural evidence suggested that the variation in the surface roughness of the Mg-Al-Zn alloy substrate determined the nucleation and growth behaviors of the coating film during the initial stage of PEO.The schematic illustration in Fig.5 shows the formation of the coating film on a single substrate with rough and smooth regions chronologically.Before the formation of the coating film,the first insulating layer,called passive film,was formed through a chemical reaction between the metal in the substrate and the ionic species in the electrolyte [25,35].The reaction forming the passive film was affected locally by the surface topography of the substrate.The Rr offered more sites for the accumulation of anions,such as OH-and SiO32-,originating from the electrolyte,owing to the greater surface area of the grooves compared to those of the hills and the Sr.This condition was shown in Fig.5a.The accumulated anions promoted the chemical reactions in the grooves,resulting in the formation of a thicker passive film on the grooves compared to those formed on the hills or in the Sr.Therefore,the resistance of the passive film against the electric field was not uniform,and the thinner passive film possessed lower resistance,as depicted in Fig.5b.The passive film on the hills was probably thinner than that in the Sr,therefore the electric charges required to induce breakdown on the hills should be lower than that required to induce breakdown in the Sr.Accordingly,plasma discharges first appeared across the Rr,particularly on the hills.After the ignition of plasma discharges on the hills,it was plausible that the discharge events occurred across the Sr before occurring in the grooves coated by the thickest passive film[36].These phenomena were confirmed by the microstructures of the coating film in both regions after the ignition of the plasma discharges.As shown in Fig.4,the coating film appeared only on the hills of the Rr and across the entire Sr.This suggested that the nucleation of the coating film on the grooves occurred later since the thickest preformed passive film had the highest electrical resistance.
The presence of exposed surfaces in the grooves which was not completely covered by the coating film,created a pathway with a relatively low electrical resistance.Thus,it was believed that the discharge activity concentrates in the grooves,producing numerous micropores in the film coating the grooves,as seen from the cross-section image in Fig.4.This local growth behavior facilitated plasma-assisted electrochemical reactions to thicken the coating film by increasing the surrounding temperature [37,38].This increased the growth rate of the coating film on the grooves and caused it to exceed the growth rate of the coating film on hills,thereby resulting in a thicker coating film,as shown in Fig.4c and schematically shown in Fig.5c.
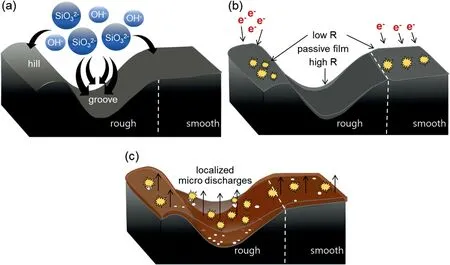
Fig.5.Schematic illustration describing the formation of the coating film on a Mg-Al-Zn alloy in chronological order: (a) the movement of anions during the formation of the passive film,(b) the breakdown of passive film during nucleation of the coating film,and (c) the growth of the coating film during the initial stage of PEO.
The present work revealed that surface topographies affected directly the ignition and proliferation behaviors of plasma discharges,which,consequently,controlled the nucleation and growth of the coating film,thus determining the overall thickness and porosity of the coating film,as confirmed by the SEM images in Figs.3 and 4.
3.3.Polarization behavior
The polarization behavior of the coating films was examined during a potentiodynamic polarization test in a 3.5wt.%NaCl solution to evaluate the electrochemical properties of the coating film,formed in each region,in a corrosive environment.The polarization curves of the coating film formed on rough and smooth regions during nucleation and growth,namely Rr-N,Rr-G,Sr-N,and Sr-G,are displayed in Fig.6,along with a bar diagram in the inset showing the polarization resistance of each sample.The electrochemical parameters,such asicorrandEcorr,iterated through Tafel extrapolation,and theRpvalues are tabulated in Table 2.
Based on the polarization curves shown in Fig.6,the values oficorrdecreased in the order of Rr-N,Sr-N,Rr-G,and Sr-G,indicating a decrease in the corrosion kinetics,which could be ascribed to the microstructure of the coating film in different regions.Meanwhile,the values ofEcorrincreased in the same order,indicating the decreasing tendency of the tested samples to be oxidized.The significant difference in theEcorrvalue of each sample was believed to be related to the structure of the coating film.The Rr-N sample achieved the lowest value ofEcorrdue to the unevenly coated metallic surface resulting from preferential,localized nucleation.
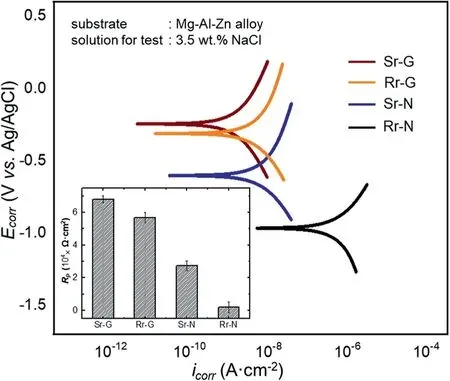
Fig.6.Potentiodynamic polarization curve of Rr and Sr samples during a test performed in 3.5wt.% NaCl solution.The inset displays a bar diagram showing the magnitudes of polarization resistance for each sample.
The ability of the coating film to isolate the underlying metal from corrosive ions in an acidic environment was closely related to the average thickness of the coating film.In the present work,the average thicknesses of the coating film in the Rr-G and Sr-G were likely comparable,although the coating film on the grooves in the Rr was somewhat thicker than that in the Sr.The Sr-G,however,demonstrated better protection performance than the Rr-G.This result was attributed to the compactness of the coating film in the Sr-G,which impeded the diffusion of corrosive ions into the substrate,thereby improving the electrochemical resistance.In contrast,the existence of numerous micro-pores in the coating film in the Rr-G,enabled corrosive ions to penetrate to the metallic substrate.These results were also supported by the polarization resistance values provided in the inset of Fig.5.The error bars corresponded to the respective standard deviations,which represented the homogeneity and uniformity of the coating structure in each region.The Sr-G achieved the highestRpand lowest deviation due to the homogeneous structure of the coating film.The evaluation of the polarization behavior showed that the coating with a low incidence of surface irregularities offered greater metallic protection in a corrosive environment because the coating grown on the smooth surface was more homogenous and uniform even in the nucleation stage compared to that grown in the rough surface.Although the coating on Rr-G was thicker than that on Sr-G,the numerous micropores concentrated in the valley allowed the corrosive ions to attack the coating easily compared to Sr with compact structure.In this study,the homogeneity of the coating film controlled by surface roughness would take the main control over the thickness in controlling the polarization behavior of the coating film.
The nucleation and growth behaviors of the coating film grown on an Al-Mg-Zn alloy during the initial stage of PEO were investigated by considering two different surface topographies of a single substrate.The breakdown phenomenon resulting in the appearance of plasma discharges occurred~5s earlier in the rough region than that in the smooth region because nucleation of the coating film favored the areas having the lowest electrical resistance,i.e.,the hills of the rough region.This result suggested that a high incidence of surface irregularities of the substrate triggered the ignition of plasma discharges,promoting early nucleation.Once plasma discharges proliferated,the growth of the coating film in the Rr was concentrated in the grooves.Although this local growth behavior resulted in a coating film that was thicker on the grooves than in the hills and in the Sr of the substrate,a greater number of micropores formed in the coating film grown on the grooves due to the localized plasma discharges.Determined by the surface roughness,the homogeneity of the coating film was found to have the main role in affecting the electrochemical resistance of the coating film against corrosive environment.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the Mid-Level Researcher National Project of the National Research Foundation (NRF)funded by the Ministry of Science and ICT,Republic of Korea (NRF-2020R1A2C2004192),and supported partly by the Competency Development Program for Industry Specialist of the Korea Institute for Advancement of Technology (KIAT)funded by the Ministry of Trade,Industry,and Energy,Republic of Korea (P0002019).
推荐访问:film Mg Al